Susceptibility of Oocytes from Gilts and Sows to Beauvericin and Deoxynivalenol and Its Relationship with Oxidative Stress
Abstract
:1. Introduction
2. Results
2.1. Oocytes from Gilts Are More Sensitive to H2O2, BEA, and DON during Maturation Than Those from Sows
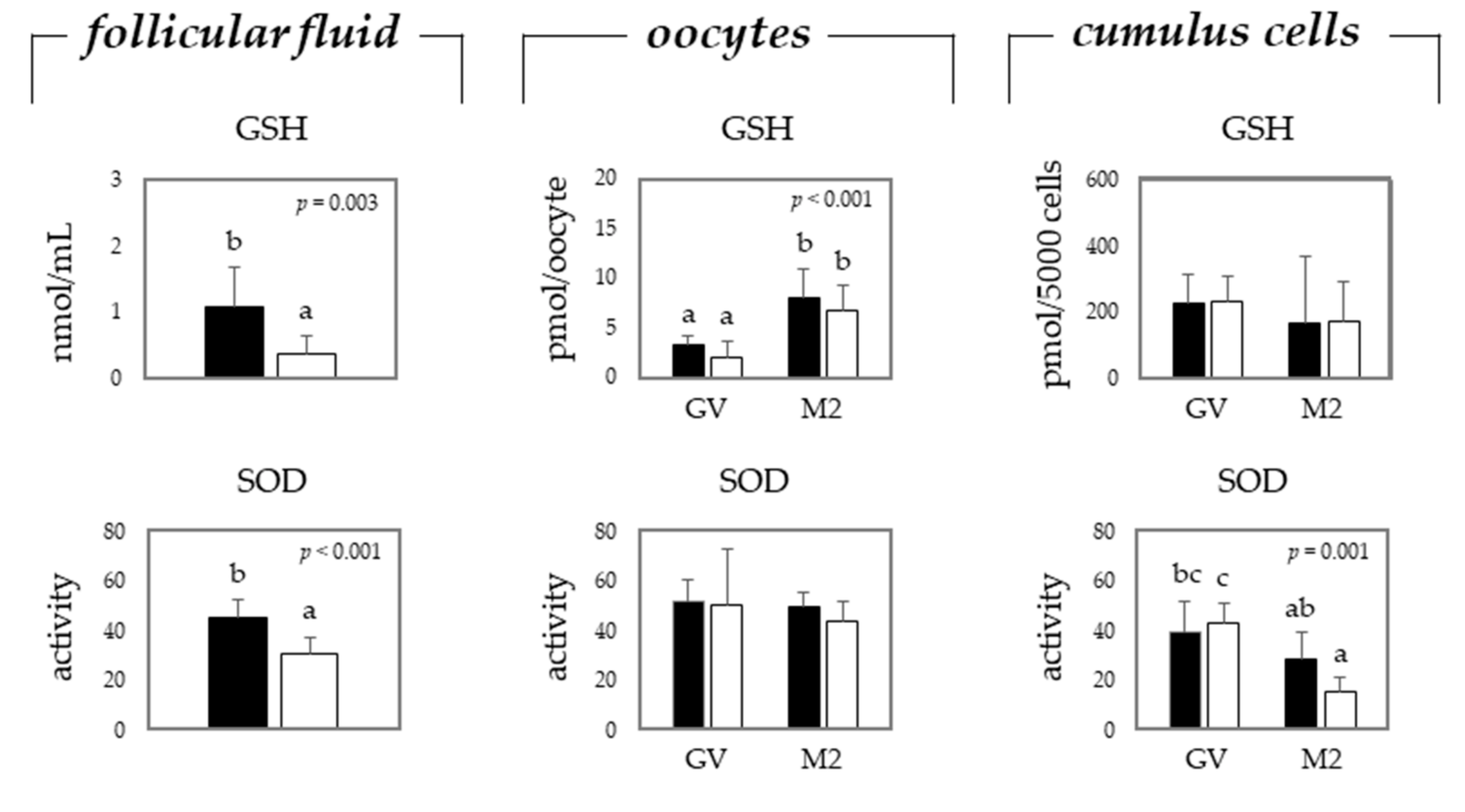
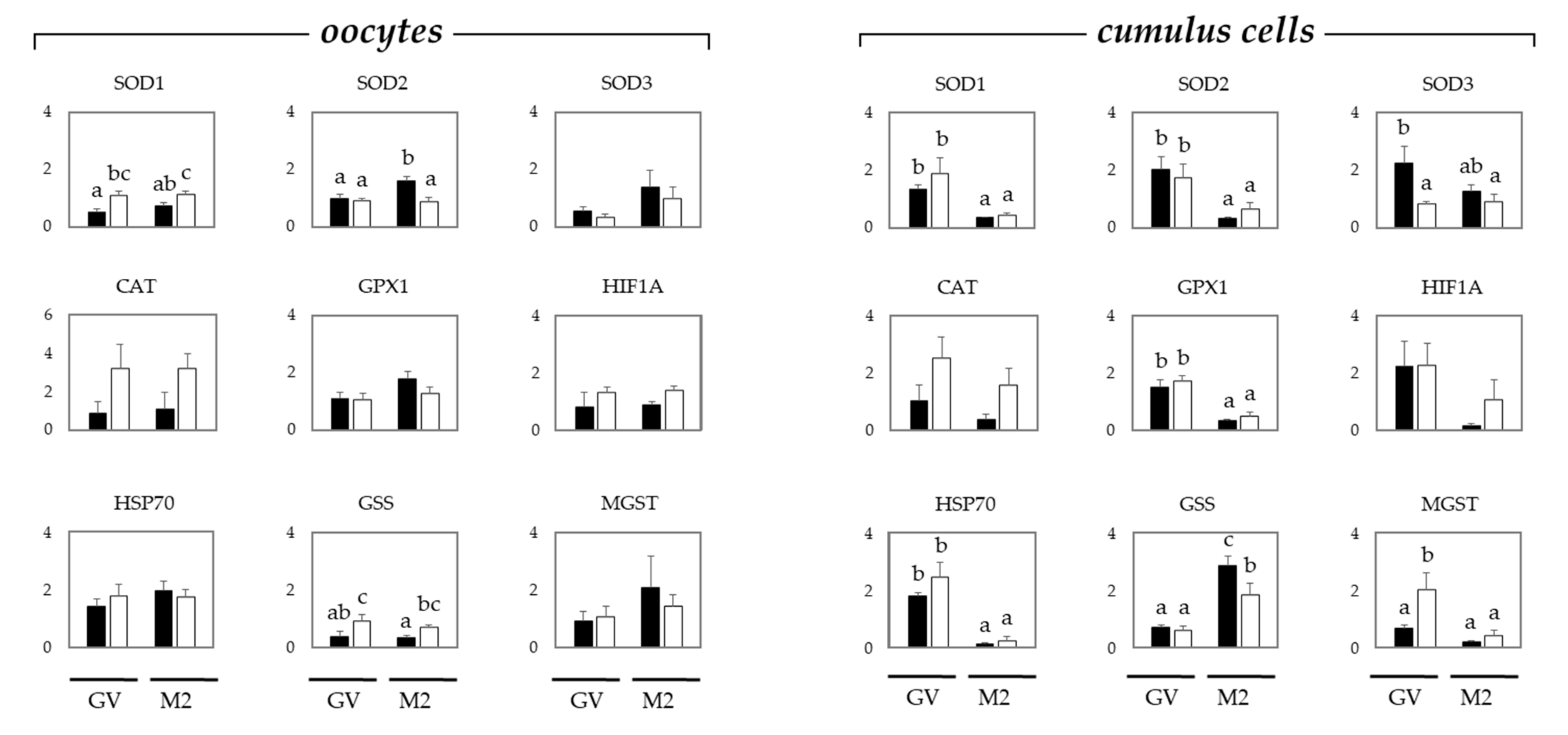
2.2. Oxidative Stress in Oocytes and Cumulus Cells Depends on the Maturation Stage and Donor Age, i.e., Gilts or Sows
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Chemicals
5.2. Selection and Culture of Cumulus Oocyte Complexes
5.3. Assessment of Cumulus Oocyte Complex Viability and Oocyte Nuclear Maturation
5.4. Markers of Antioxidant Capacity and Oxidative Stress
5.5. Quantitative Reverse Transcription-PCR
5.6. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ANOVA | Analysis of variance |
| AOH | Alternariol |
| B2M | β-2-microglobulin |
| BACT | β-Actin |
| BEA | Beauvericin |
| CAT | Catalase |
| cDNA | Complement deoxyribonucleic acid |
| COCs | Cumulus oocyte complexes |
| Cu/ZnSOD | Copper/zink superoxide dismutase |
| DAPI | 4′,6-diamidino-2-phenylindole |
| DMSO | Dimethyl sulfoxide |
| dNTP | Deoxyribonucleotide triphosphate |
| DON | Deoxynivalenol |
| EthD-1 | Ethidium homodimer-1 |
| FF | Follicular fluid |
| FSH | Follicle-stimulating hormone |
| GAPDH | Glyceraldehyde 3-phosphate dehydrogenase |
| GLRX2 | Glutaredoxin 2 |
| GPx | Glutathione peroxidase |
| GPX1 | Glutathione peroxidase 1 |
| GSH | Glutathione |
| GSR | Glutathione reductase |
| GSS | Glutathione synthetase |
| GV | Germinal vesicle |
| HEPES | 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid |
| HIF1A | Hypoxia-inducible factor 1 |
| HSP70 | Heat shock protein 70 |
| IU | International units |
| M2 | Metaphase II |
| MGST | Microsomal glutathione S-transferase |
| MnSOD | Manganese superoxide dismutase |
| OMM | Oocyte maturation medium |
| PBS | Phosphate buffer saline |
| PCR | Polymerase chain reaction |
| PDIA4 | Protein disulfide isomerase 4 |
| PGK1 | Phosphoglycerate kinase 1 |
| qRT-PCR | quantitative reverse transcription-polymerase chain reaction |
| RFU | Relative fluorescence units |
| RNA | Ribonucleic acid |
| ROS | Reactive oxygen species |
| -RT | Minus reverse transcriptase |
| SEM | Standard error of the mean |
| SOD | Superoxide dismutase |
| SOD1 | Superoxide dismutase 1 |
| SOD2 | Superoxide dismutase 2 |
| SOD3 | Superoxide dismutase 3 |
| TEAC | Total equivalent antioxidant activity |
| TXNRD1 | Thioredoxin reductase |
| ZEN | Zearalenone |
References
- Alm, H.; Greising, T.; Brussow, K.P.; Torner, H.; Tiemann, U. The Influence of the Mycotoxins Deoxynivalenol and Zearalenol on in vitro Maturation of Pig Oocytes and in vitro Culture of Pig Zygotes . Toxicol. In Vitro 2002, 16, 643–648. [Google Scholar] [CrossRef]
- Malekinejad, H.; Schoevers, E.J.; Daemen, I.J.; Zijlstra, C.; Colenbrander, B.; Fink-Gremmels, J. Exposure of Oocytes to the Fusarium Toxins Zearalenone and Deoxynivalenol Causes Aneuploidy and Abnormal Embryo Development in Pigs. Biol. Reprod. 2007, 77, 840–847. [Google Scholar] [CrossRef] [Green Version]
- Han, J.; Wang, Q.C.; Zhu, C.C.; Liu, J.; Zhang, Y.; Cui, X.S.; Kim, N.H.; Sun, S.C. Deoxynivalenol Exposure Induces Autophagy/Apoptosis and Epigenetic Modification Changes during Porcine Oocyte Maturation. Toxicol. Appl. Pharmacol. 2016, 300, 70–76. [Google Scholar] [CrossRef]
- Schoevers, E.J.; Fink-Gremmels, J.; Colenbrander, B.; Roelen, B.A. Porcine Oocytes are most Vulnerable to the Mycotoxin Deoxynivalenol during Formation of the Meiotic Spindle. Theriogenology 2010, 74, 968–978. [Google Scholar] [CrossRef]
- Schoevers, E.J.; Santos, R.R.; Fink-Gremmels, J.; Roelen, B.A. Toxicity of Beauvericin on Porcine Oocyte Maturation and Preimplantation Embryo Development. Reprod. Toxicol. 2016, 65, 159–169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schoevers, E.J.; Santos, R.R.; Roelen, B.A.J. Alternariol Disturbs Oocyte Maturation and Preimplantation Development. Mycotoxin. Res. 2020, 36, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Schoevers, E.J.; Santos, R.R.; Colenbrander, B.; Fink-Gremmels, J.; Roelen, B.A. Transgenerational Toxicity of Zearalenone in Pigs. Reprod. Toxicol. 2012, 34, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Benthem de Grave, X.; Saltzmann, J.; Laurain, J.; Rodriguez, M.A.; Molist, F.; Dänicke, S.; Santos, R.R. Transmission of Zearalenone, Deoxynivalenol, and Their Derivatives from Sows to Piglets during Lactation. Toxins 2021, 13, 37. [Google Scholar] [CrossRef]
- Pestka, J.J. Deoxynivalenol: Mechanisms of Action, Human Exposure, and Toxicological Relevance. Arch. Toxicol. 2010, 84, 663–679. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.R.; Lau, A.S.; Pestka, J.J. Role of Double-stranded RNA-activated Protein Kinase R (PKR) in Deoxynivalenol-induced Ribotoxic Stress Response. Toxicol. Sci. 2003, 74, 335–344. [Google Scholar] [CrossRef] [Green Version]
- Payros, D.; Alassane-Kpembi, I.; Pierron, A.; Loiseau, N.; Pinton, P.; Oswald, I.P. Toxicology of Deoxynivalenol and Its Acetylated and Modified Forms. Arch. Toxicol. 2016, 90, 2931–2957. [Google Scholar] [CrossRef]
- Caloni, F.; Fossati, P.; Anadon, A.; Bertero, A. Beauvericin: The Beauty and the Beast. Environ. Toxicol. Pharmacol. 2020, 75, 103349. [Google Scholar] [CrossRef] [PubMed]
- Olleik, H.; Nicoletti, C.; Lafond, M.; Courvoisier-Dezord, E.; Xue, P.; Hijazi, A.; Baydoun, E.; Perrier, J.; Maresca, M. Comparative Structure-activity Analysis of the Antimicrobial Activity, Cytotoxicity, and Mechanism of Action of the Fungal Cyclohexadepsipeptides Enniatins and Beauvericin. Toxins 2019, 11, 514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mallebrera, B.; Prosperini, A.; Font, G.; Ruiz, M.J. In vitro Mechanisms of Beauvericin Toxicity: A Review. Food Chem. Toxicol. 2018, 111, 537–545. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Gupta, S.; Sharma, R.K. Role of Oxidative Stress in Female Reproduction. Reprod. Biol. Endocrinol. 2005, 3, 28. [Google Scholar] [CrossRef] [Green Version]
- Pisoschi, A.M.; Pop, A. The Role of Antioxidants in the Chemistry of Oxidative Stress: A Review. Eur. J. Med. Chem. 2015, 97, 55–74. [Google Scholar] [CrossRef] [PubMed]
- El Golli-Bennour, E.; Bacha, H. Hsp70 Expression as Biomarkers of Oxidative Stress: Mycotoxins’ Exploration. Toxicology 2011, 287, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Yalu, R.; Oyesiji, A.E.; Eisenberg, I.; Imbar, T.; Meidan, R. HIF1A-dependent Increase in Endothelin 2 Levels in Granulosa Cells: Role of Hypoxia, LH/cAMP, and Reactive Oxygen Species. Reproduction 2015, 149, 11–20. [Google Scholar] [CrossRef] [Green Version]
- Osselaere, A.; Santos, R.; Hautekiet, V.; De Backer, P.; Chiers, K.; Ducatelle, R.; Croubels, S. Deoxynivalenol Impairs Hepatic and Intestinal Gene Expression of Selected Oxidative Stress, Tight Junction and Inflammation Proteins in Broiler Chickens, but Addition of an Adsorbing Agent Shifts the Effects to the Distal Parts of the Small Intestine. PLoS ONE 2013, 8, e69014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, S.; Zheng, L.; Wan, M.; Niu, J.; Liu, Y.; Tian, L. Effect of Deoxynivalenol on Growth Performance, Histological Morphology, Anti-oxidative Ability and Immune Response of Juvenile Pacific White Shrimp, Litopenaeus vannamei. Fish Shellfish Immunol. 2018, 82, 442–452. [Google Scholar] [CrossRef]
- Marchal, R.; Feugang, J.M.; Perreau, C.; Venturi, E.; Terqui, M.; Mermillod, P. Meiotic and Developmental Competence of Prepubertal and Adult Swine Oocytes. Theriogenology 2001, 56, 17–29. [Google Scholar] [CrossRef]
- Pinkert, C.A.; Kooyman, D.L.; Baumgartner, A.; Keisler, D.H. In vitro Development of Zygotes from Superovulated Prepubertal and Mature Gilts. J. Reprod. Fert. 1989, 87, 63–66. [Google Scholar] [CrossRef] [Green Version]
- Koenig, J.L.F.; Stormshak, F. Cytogenetic Evaluation of Ova from Pubertal and Third-estrous Gilts. Biol. Reprod. 1993, 49, 1158–1162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, Y.; Wheeler, M.B.; Krisher, R.L. Disrupted Redox Homeostasis and Aberrant Redox Gene Expression in Porcine Oocytes Contribute to Decreased Developmental Competence. Biol. Reprod. 2012, 87, 78. [Google Scholar] [CrossRef] [PubMed]
- Knox, R.V. Physiology and Endocrinology Symposium: Factors Influencing Follicle Development in Gilts and Sows and Management Strategies Used to Regulate Growth for Control of Estrus and Ovulation. J. Anim. Sci. 2019, 97, 1433–1445. [Google Scholar] [CrossRef] [PubMed]
- Ambekar, A.S.; Nirujogi, R.S.; Srikanth, S.M.; Chavan, S.; Kelkar, D.S.; Hinduja, I.; Zaveri, K.; Keshava Prasad, T.S.; Harsha, H.C.; Pandey, A.; et al. Proteomic Analysis of Human Follicular Fluid: A New Perspective Towards Understanding Folliculogenesis. J. Proteom. 2013, 87, 68–77. [Google Scholar] [CrossRef]
- Albertini, D.F.; Combelles, C.M.; Benecchi, E.; Carabatsos, M.J. Cellular Basis for Paracrine Regulation of Ovarian Follicle Development. Reproduction 2001, 121, 647–653. [Google Scholar] [CrossRef]
- Da Broi, M.G.; Giorgi, V.S.; Wang, F.; Keefe, D.L.; Albertini, D.; Navarro, P.A. Influence of Follicular Fluid and Cumulus Cells on Oocyte Quality Clinical Implications. J. Assist. Reprod. Genet. 2018, 35, 735–751. [Google Scholar] [CrossRef]
- Paczkowski, M.; Yuan, Y.; Fleming-Waddell, J.; Bidwell, C.A.; Spurlock, D.; Krisher, R.L. Alterations in the Transcriptome of Porcine Oocytes Derived from Prepubertal and Cyclic Females is Associated with Developmental Potential. J. Anim. Sci. 2011, 89, 3561–3571. [Google Scholar] [CrossRef]
- Furnus, C.C.; de Matos, D.G.; Moses, D.F. Cumulus Expansion during in vitro Maturation of Bovine Oocytes: Relationship with Intracellular Glutathione Level and Its Role on Subsequent Embryo Development. Mol. Reprod. Dev. 1998, 51, 76–83. [Google Scholar] [CrossRef]
- Schoevers, E.J.; Colenbrander, B.; Roelen, B.A. Developmental Stage of the Oocyte during Antral Follicle Growth and Cumulus Investment Determines in vitro Embryo Development of Sow Oocytes. Theriogenology 2007, 67, 1108–1122. [Google Scholar] [CrossRef] [PubMed]
- Pawlak, P.; Warzych, E.; Hryciuk, M.; Lechniak, D. Transcript Abundance, Glutathione and Apoptosis Levels Differ Between Porcine Oocytes Collected from Prepubertal and Cyclic Gilts. Theriogenology 2015, 84, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Freitas, C.; Neto, A.C.; Matos, L.; Silva, E.; Ribeiro, A.; Silva Carvalho, J.L.; Almeida, H. Follicular Fluid Redox Involvement for Ovarian Follicle Growth. J. Ovarian Res. 2017, 10, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Da Broi, M.G.; Albuquerque, F.O.; Andrade, A.Z.; Cardoso, R.L.; Jordao Junior, A.A.; Navarro, P.A. Increased Concentration of 8-hydroxy-2′-deoxyguanosine in Follicular Fluid of Infertile Women with Endometriosis. Cell Tissue Res. 2016, 366, 231–242. [Google Scholar] [CrossRef]
- Liu, R.H.; Li, Y.H.; Jiao, L.H.; Wang, X.N.; Wang, H.; Wang, W.H. Extracellular and Intracellular Factors Affecting Nuclear and Cytoplasmic Maturation of Porcine Oocytes Collected from Different Sizes of Follicles. Zygote 2002, 10, 253–260. [Google Scholar] [CrossRef]
- Bisseling, J.G.; Knapen, M.F.; Goverde, H.J.; Mulder, T.P.; Peters, W.H.; Willemsen, W.N.; Thomas, C.M.; Steegers, E.A. Glutathione S-transferases in Human Ovarian Follicular Fluid. Fertil. Steril. 1997, 68, 907–911. [Google Scholar] [CrossRef] [Green Version]
- Basini, G.; Simona, B.; Santini, S.E.; Grasselli, F. Reactive Oxygen Species and Anti-oxidant Defences in Swine Follicular Fluids. Reprod. Fertil. Dev. 2008, 20, 269–274. [Google Scholar] [CrossRef]
- Sabatini, L.; Wilson, C.; Lower, A.; Al-Shawaf, T.; Grudzinkas, J.G. Superoxide Dismutase Activity in Human Follicular Fluid after Controlled Ovarian Hyperstimulation in Women Undergoing in vitro Fertilization. Fertil. Steril. 1999, 72, 1027–1034. [Google Scholar] [CrossRef]
- Santos, R.R.; Schoevers, E.J.; Wu, X.; Roelen, B.A.J.; Fink-Gremmels, J. The Protective Effect of Follicular Fluid against the Emerging Mycotoxins Alternariol and Beauvericin. World Mycotoxin J. 2015, 8, 445–450. [Google Scholar] [CrossRef]
- Von Mengden, L.; Klamt, F.; Smitz, J. Redox Biology of Human Cumulus Cells: Basic Concepts, Impact on Oocyte Quality, and Potential Clinical Use. Antioxid. Redox Signal. 2020, 10, 522–535. [Google Scholar] [CrossRef] [Green Version]
- Lonergan, P.; Gutierrez-Adan, A.; Rizos, D.; Pintado, B.; de la Fuente, J.; Boland, M.P. Relative Messenger RNA Abundance in Bovine Oocytes Collected in vitro or in vivo before and 20 hr after the Preovulatory Luteinizing Hormone Surge. Mol. Reprod. Dev. 2003, 66, 297–305. [Google Scholar] [CrossRef]
- Combelles, C.M.; Gupta, S.; Agarwal, A. Could Oxidative Stress Influence the in vitro Maturation of Oocytes? Reprod. Biomed. Online 2009, 18, 868–880. [Google Scholar] [CrossRef]
- Mallebrera, B.; Juan-Garcia, A.; Font, G.; Ruiz, M.J. Mechanisms of Beauvericin Toxicity and Antioxidant Cellular Defense. Toxicol. Lett. 2016, 246, 28–34. [Google Scholar] [CrossRef]
- Wu, Q.-H.; Wang, X.; Yang, W.; Nussler, A.K.; Xiong, L.-Y.; Kuca, K.; Dohnal, V.; Zhang, X.-J.; Yuan, Z.-H. Oxidative stress-mediated Cytotoxicity and Metabolism of T-2 Toxin and Deoxynivalenol in Animals and Humans: An Update. Arch. Toxicol. 2014, 88, 1309–1326. [Google Scholar] [CrossRef]
- Keira, M.; Nishihira, J.; Ishibashi, T.; Tanaka, T.; Fujimoto, S. Identification of a Molecular Species in Porcine Ovarian Luteal Glutathione S-transferase and Its Hormonal Regulation by Pituitary Gonadotropins. Arch. Biochem. Biophys. 1994, 308, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Hidaka, T.; Fukumoto, Y.; Yamamoto, S.; Ogata, Y.; Horiuchi, T. Variations in Bovine Embryo Production between Individual Donors for OPU-IVF are Closely Related to Glutathione Concentrations in Oocytes during in vitro Maturation. Theriogenology 2018. [Google Scholar] [CrossRef] [PubMed]
- Jiao, G.Z.; Cao, X.Y.; Cui, W.; Lian, H.Y.; Miao, Y.L.; Wu, X.F.; Han, D.; Tan, J.H. Developmental Potential of Prepubertal Mouse Oocytes is Compromised Due Mainly to Their Impaired Synthesis of Glutathione. PLoS ONE 2013, 8, e58018. [Google Scholar] [CrossRef] [Green Version]
- Paczkowski, M.; Krisher, R. Aberrant Protein Expression is Associated with Decreased Developmental Potential in Porcine Cumulus-oocyte Complexes. Mol. Reprod. Dev. 2010, 77, 51–58. [Google Scholar] [CrossRef]
- Maedomari, N.; Kikuchi, K.; Noguchi, J.; Kaneko, H.; Ohnuma, K.; Nakai, M.; Shino, M.; Nagai, T.; Kashiwazaki, N. Cytoplasmic Glutathione Regulated by Cumulus Cells during Porcine Oocyte Maturation Affects Fertilization and Embryonic Development in vitro. Theriogenology 2007, 67, 983–993. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Jin, Y.; Wu, S.; Yu, H.; Zhao, Y.; Fang, H.; Shen, J.; Zhou, C.; Fu, Y.; Li, R.; et al. Deoxynivalenol Induces Oxidative Stress, Inflammatory Response and Apoptosis in Bovine Mammary Epithelial Cells. J. Anim. Physiol. Anim. Nutr. 2019, 103, 1663–1674. [Google Scholar] [CrossRef]
- Juan-Garcia, A.; Carbone, S.; Ben-Mahmoud, M.; Sagratini, G.; Manes, J. Beauvericin and Ochratoxin A Mycotoxins Individually and Combined in HepG2 Cells Alter Lipid Peroxidation, Levels of Reactive Oxygen Species and Glutathione. Food Chem. Toxicol. 2020, 139, 111247. [Google Scholar] [CrossRef]
- Santos, R.R.; Schoevers, E.J.; Roelen, B.A.J.; Fink-Gremmels, J. Mycotoxins and Female Reproduction: In vitro Approaches. World Mycotoxin J. 2013, 6, 245–253. [Google Scholar] [CrossRef]
- Santos, R.R.; Schoevers, E.J.; Roelen, B.A.J. Usefulness of Bovine and Porcine IVM/IVF Models for Reproductive Toxicology. Reprod. Biol. Endocrinol. 2014, 12, 117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Habschied, K.; Kanižai Šarić, G.; Krstanović, V.; Mastanjević, K. Mycotoxins—Biomonitoring and Human Exposure. Toxins 2021, 13, 113. [Google Scholar] [CrossRef]
- Goyarts, T.; Danicke, S. Bioavailability of the Fusarium Toxin Deoxynivalenol (DON) from Naturally Contaminated Wheat for the Pig. Toxicol. Lett. 2006, 163, 171–182. [Google Scholar] [CrossRef]
- Meky, F.A.; Turner, P.C.; Ashcroft, A.E.; Miller, J.D.; Qiao, Y.L.; Roth, M.J.; Wild, C.P. Development of a Urinary Biomarker of Human Exposure to Deoxynivalenol. Food. Chem. Toxicol. 2003, 41, 265–273. [Google Scholar] [CrossRef]
- Serrano, A.B.; Capriotti, A.L.; Cavaliere, C.; Piovesana, S.; Samperi, R.; Ventura, S.; Lagana, A. Development of a Rapid LC-MS/MS Method for the Determination of Emerging Fusarium Mycotoxins Enniatins and Beauvericin in Human Biological Fluids. Toxins 2015, 7, 3554–3571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schoevers, E.J.; Kidson, A.; Verheijden, J.H.; Bevers, M.M. Effect of Follicle-stimulating Hormone on Nuclear and Cytoplasmic Maturation of Sow Oocytes in vitro. Theriogenology 2003, 59, 2017–2028. [Google Scholar] [CrossRef]
- Wang, X.; Zhu, X.; Liang, X.; Xu, H.; Liao, Y.; Lu, K.; Lu, S. Effects of Resveratrol on in vitro Maturation of Porcine Oocytes and Subsequent Early Embryonic Development Following Somatic Cell Nuclear Transfer. Reprod. Domest. Anim. 2019, 54, 1195–1205. [Google Scholar] [CrossRef] [PubMed]
- Pereira, B.A.; Zangeronimo, M.G.; Castillo-Martin, M.; Gadani, B.; Chaves, B.R.; Rodriguez-Gil, J.E.; Bonet, S.; Yeste, M. Supplementing Maturation Medium with Insulin Growth Factor I and Vitrification-Warming Solutions With Reduced Glutathione Enhances Survival Rates and Development Ability of in vitro Matured Vitrified-Warmed Pig Oocytes. Front. Physiol. 2019, 9, 1894. [Google Scholar] [CrossRef] [Green Version]
- Kwiatkowska, J.; Wasowska, B.; Gilun, P. Expression of Hypoxia Inducible Factor 1α and Antioxidant Enzymes: Superoxide Dismutases-1 and -2 in Ischemic Porcine Endometrium. Reprod. Biol. 2017, 17, 289–293. [Google Scholar] [CrossRef] [PubMed]
- Chmielewska, M.; Tosiewicz, K.; Podlasz, P.; Wasowicz, K. The Expression of Mitochondrial, Cytoplasmic and Extracellular Superoxide Dismutase in the Colonic Wall of Pigs Suffering from Swine Dysenteria. Pol. J. Vet. Sci. 2013, 16, 463–467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Treatment | Age | Maturation Rate | Degeneration Rate | ||
|---|---|---|---|---|---|
| Control | Gilt | 82.9 | c | 4.8 | a |
| Control | Sow | 86.9 | c | 2.4 | a |
| 10 μmol/L | Gilt | 65.7 | c | 9.1 | a |
| 10 μmol/L | Sow | 86.8 | c | 2.5 | a |
| 50 μmol/L | Gilt | 35.7 | b | 26.8 | b |
| 50 μmol/L | Sow | 71.9 | c | 9.7 | a |
| 100 μmol/L | Gilt | 7.7 | a | 88.1 | c |
| 100 μmol/L | Sow | 65.5 | c | 30.4 | b |
| Control | 84.9 | 3.6 | |||
| 10 μmol/L | 76.2 | 5.8 | |||
| 50 μmol/L | 53.8 | 18.2 | |||
| 100 μmol/L | 36.6 | 59.2 | |||
| Gilt | 48.0 | 32.2 | |||
| Sow | 77.8 | 11.2 | |||
| Effect of: | p-value | SEM | p-value | SEM | |
| Treatment × Age | 0.017 | 7.60 | <0.001 | 3.34 | |
| Age | <0.001 | 3.80 | <0.001 | 1.67 | |
| Treat | <0.001 | 5.38 | <0.001 | 2.36 | |
| Treatment Linear | 0.006 | <0.001 | |||
| Treatment Quadratic | 0.494 | 0.072 | |||
| Treatment | Age | Maturation Rate | Degeneration Rate | ||
|---|---|---|---|---|---|
| Control | Gilt | 74.0 | cd | 11.7 | a |
| Control | Sow | 77.4 | cd | 1.5 | a |
| 0.5 μmol/L | Gilt | 46.8 | b | 28.1 | b |
| 0.5 μmol/L | Sow | 82.0 | d | 2.7 | a |
| 2.5 μmol/L | Gilt | 4.8 | a | 91.6 | c |
| 2.5 μmol/L | Sow | 72.3 | cd | 9.7 | a |
| 5.0 μmol/L | Gilt | 0.0 | a | 100.0 | c |
| 5.0 μmol/L | Sow | 64.8 | c | 11.1 | a |
| Control | 75.7 | 6.6 | |||
| 0.5 μmol/L | 64.4 | 15.5 | |||
| 2.5 μmol/L | 38.6 | 50.7 | |||
| 5.0 μmol/L | 32.4 | 55.5 | |||
| Gilt | 31.4 | 57.9 | |||
| Sow | 74.1 | 6.2 | |||
| Effect of: | p-value | SEM | p-value | SEM | |
| Treatment × Age | <0.001 | 5.08 | <0.001 | 3.59 | |
| Age | <0.001 | 2.54 | <0.001 | 1.80 | |
| Treat | <0.001 | 3.59 | <0.001 | 2.54 | |
| Treatment Linear | 0.016 | 0.010 | |||
| Treatment Quadratic | 0.857 | 0.319 | |||
| Treatment | Age | Maturation Rate | Degeneration Rate | ||
|---|---|---|---|---|---|
| Control | Gilt | 67.5 | 13.3 | ||
| Control | Sow | 73.2 | 9.1 | ||
| 0.02 μmol/L | Gilt | 37.1 | 12.7 | ||
| 0.02 μmol/L | Sow | 63.5 | 7.5 | ||
| 0.2 μmol/L | Gilt | 22.9 | 29.7 | ||
| 0.2 μmol/L | Sow | 59.7 | 8.8 | ||
| 2.0 μmol/L | Gilt | 8.7 | 35.4 | ||
| 2.0 μmol/L | Sow | 42.5 | 7.5 | ||
| Control | 70.4 | c | 11.2 | ||
| 0.02 μmol/L | 50.3 | b | 10.1 | ||
| 0.2 μmol/L | 41.3 | b | 19.2 | ||
| 2.0 μmol/L | 25.6 | a | 21.5 | ||
| Gilt | 34.0 | a | 22.8 | b | |
| Sow | 59.7 | b | 8.2 | a | |
| Effect of: | p-value | SEM | p-value | SEM | |
| Treatment × Age | 0.178 | 7.17 | 0.089 | 5.04 | |
| Age | <0.001 | 3.58 | 0.001 | 2.52 | |
| Treat | <0.001 | 5.07 | 0.101 | 3.57 | |
| Treatment Linear | 0.026 | 0.798 | |||
| Treatment Quadratic | 0.863 | 0.885 | |||
| Gene | Protein | Sequence | Product Size (bp) | Annealing Temperature (°C) | Gene Bank Accession Number |
|---|---|---|---|---|---|
| Reference | |||||
| B2M | β-2-microglobulin | F: 5′-TTCACACCGCTCCAGTAG-3′ R: 5′-CCAGATACATAGCAGTTCAGG-3′ | 166 | 60.0 | NM_213978 |
| BACT | β-Actin | F: 5′-CATCACCATCGGCAACGAGC-3′ R: 5′-TAGAGGTCCTTGCGGATGTC-3′ | 141 | 56.0 | AY550069 |
| PGK1 | Phosphoglycerate kinase 1 | F: 5′-AGATAACGAACAACCAGAGG-3′ R: 5′-TGTCAGGCATAGGGATACC-3′ | 126 | 56.0 | AY677198 |
| GAPDH | Glyceraldehyde 3-phosphate dehydrogenase | F: 5′ -TCGGAGTGAACGGATTTG-3′ R: 5′-CCTGGAAGATGGTGATGG-3′ | 219 | 61.0 | AF017079 |
| Target | |||||
| CAT [59] | Catalase | F: 5′-ATGTGCAGGCTGGATCTCAC-3′ R: 5′-GCACAGGAGAATCTTGCATCC-3′ | 155 | 55.0 | XM_021081498.1 |
| GPX1 [60] | Glutathione peroxidase 1 | F: 5′-CAAGAATGGGGAGATCCTGA-3′ R: 5′-GTCA TTGCGACACACTGGAG-3′ | 217 | 64.5 | NM_2142201.1 |
| GSS | Glutathione synthetase | F: 5′-TGGTTTACTTCCGGGATGGC-3′ R: 5′-CGCCTACTCTGCTTAGCTCC-3′ | 159 | 64.5 | NM_001244625.1 |
| HIF1A [61] | Hypoxia inducible factor 1 alpha | F: 5′-TCAGCTATTTGCGTGTGAGG-3′ R: 5′-TTCACAAATCAGACCAAGC-3′ | 479 | 61.0 | NM_001123124.1 |
| HSP70 [60] | Heat shock protein 70 | F: 5′-ATGTCCGCTGCAAGAGAAGT-3′ R: 5′-GGCGTCAAACACGGTATTCT-3′ | 216 | 64.5 | NM_001123127.1 |
| MGST | Microsomal glutathione S-transferase 1 | F: 5′-CGAGGAATTAAGATAGAGAAAGCCT-3′ R: 5′-TGGGCCCATTTGGAAAATACTGA-3′ | 247 | 64.5 | NM_214300.2 |
| SOD1 [59] | Superoxide dismutase 1 | F: 5′-CGAGCTGAAGGGAGAGAAGA-3′ R: 5′-ACATTGCCCAGGTCTCCAAC-3′ | 199 | 64.5 | NM_001190422.1 |
| SOD2 [61] | Superoxide dismutase 2 | F: 5′-CCCTGGAGCCGCACATC-3′ R: 5′-TTTTTCAGCGCCTCCTG-3′ | 115 | 64.5 | NM_214127.2 |
| SOD3 [62] | Superoxide dismutase 3 | R: 5′-ACTCCTGCCATGCTGACG-3′ F: 5′-TGCCAGATCTCCGTCACTTT-3′ | 140 | 64.5 | DQ915492.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schoevers, E.J.; Santos, R.R.; Roelen, B.A.J. Susceptibility of Oocytes from Gilts and Sows to Beauvericin and Deoxynivalenol and Its Relationship with Oxidative Stress. Toxins 2021, 13, 260. https://doi.org/10.3390/toxins13040260
Schoevers EJ, Santos RR, Roelen BAJ. Susceptibility of Oocytes from Gilts and Sows to Beauvericin and Deoxynivalenol and Its Relationship with Oxidative Stress. Toxins. 2021; 13(4):260. https://doi.org/10.3390/toxins13040260
Chicago/Turabian StyleSchoevers, Eric J., Regiane R. Santos, and Bernard A. J. Roelen. 2021. "Susceptibility of Oocytes from Gilts and Sows to Beauvericin and Deoxynivalenol and Its Relationship with Oxidative Stress" Toxins 13, no. 4: 260. https://doi.org/10.3390/toxins13040260
APA StyleSchoevers, E. J., Santos, R. R., & Roelen, B. A. J. (2021). Susceptibility of Oocytes from Gilts and Sows to Beauvericin and Deoxynivalenol and Its Relationship with Oxidative Stress. Toxins, 13(4), 260. https://doi.org/10.3390/toxins13040260







